Background: The decision to re-induce patients with newly diagnosed acute myeloid leukemia (AML) currently relies on morphological evaluation of leukemia blasts in post treatment bone marrow, with more than 5% bone marrow blast counts indicating necessity of re-induction therapy. Our previous study showed that changes in Leukemia Stem Cell (LSC) subsets, identified by CLL1 (CD371) or CD45RA as specific markers, are highly correlated with therapeutic outcome. In this study, we focus on newly diagnosed AML patients undergoing induction therapy to determine whether information gained from LSC subset analysis can aid in decision making for re-induction therapy.
Methods: We analyzed flow cytometry data from bone marrow aspirate in patients with newly diagnosed AML of all types except acute promyelocytic leukemia (M3) before and after induction therapy from a single institution. “Leukemic blasts” were identified by morphology and leukemia-associated immunophenotype by Multicolor Flow cytometry (MFC). Hematopoietic stem cells (HSCs) gating was based on CD45 dim/SSC and CD34 +CD38 low/- expression. Leukemic stem cells (LSC) subset was analyzed using % of CD371 (CLL-1) or CD45RA positive cells over total HSCs (%CLL1 +/CD34 +CD38 - or %CD45RA +/CD34 +CD38 -). Newly diagnosed AML with >50% (CLL1 +/CD34 +CD38 - or CD45RA +/CD34 +CD38 -) on diagnosis was selected for further study. Fold change of LSC subset was calculated using LSC percentage post-induction divided by LSC percentage on diagnosis. Chart review was conducted to determine correlation between LSC subset change, bone marrow blasts count and clinical course. Mann-Whitney test was used for statistical analysis.
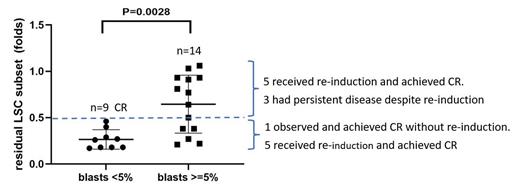
Results: Forty-eight newly diagnosed AML (non-M3) cases, who received at least one cycle of induction therapy with both pre- and post-treatment bone marrow biopsy were reviewed. Twenty-seven cases were identified with CLL1 +/CD34 +CD38 - or CD45RA +/CD34 +CD38 - above the 50% threshold on diagnosis. Four cases were later excluded due to inadequate CD34 +CD38 - cellular events on flow cytometry (<20) in post-treatment bone marrow samples. As shown in figure 1, in all the 9 patients with post induction blasts counts of less than 5% (complete remission, CR), post induction LSC subset is reduced to less than 0.5× of LSC subset on diagnosis. Thus, post induction LSC subset less than 0.5× of diagnosis is used as cut off for “adequate reduction”. In 14 patients with residual blasts of more than 5%, there is a wide range of LSC subset change. Interestingly, 6 of them showed adequate reduction of LSC subsets, similar to patients who achieved CR. Among them, one was observed and showed CR on repeat bone marrow biopsy at a later time point without further therapy. The other 5 patients were given re-induction and achieved CR after a second cycle. Eight patients with residual blasts did not achieve adequate reduction of LSC subset (residual LSC subset > 0.5× of diagnosis). Five achieved CR on subsequent re-induction. Three had persistent disease and failed to achieve CR despite 3 cycles of induction therapy.
Conclusion: In newly diagnosed AML with high percent of CLL1 +/CD34 +CD38 - or CD45RA +/CD34 +CD38 - LSC subset on diagnosis, monitoring LSC subset offers additional information regarding efficacy of induction therapy. All the patients with CR showed residual LSC subset of 0.5-fold or less compared to LSC subset on diagnosis (defined as “adequate reduction” of LSC subset). For patients who did not achieve remission but adequately reduced LSC subset, which indicates efficacy of induction therapy, observation with repeat bone marrow biopsy, or re-induction with similar regimen can be considered. For patients with no adequate reduction of LSC subset, intensified chemotherapy or alternative therapy with different mechanism of action needs to be considered to maximize the possibility of achieving CR on re-induction. A multi-institutional study with more patients is needed to confirm these findings and validate these LSC biomarkers for this important treatment decision making in AML.
Disclosures
No relevant conflicts of interest to declare.